Elbow Torque Tracker for UCL Surgery Recovery
Cornell DEBUT
Situation
Improve university-level product development lifecycle to improve translation into entrepreneurial ventures (from previous SteadyStride learnings)
Train new members on integrating electromechanical design into medical products in a documented, well-regulated environment
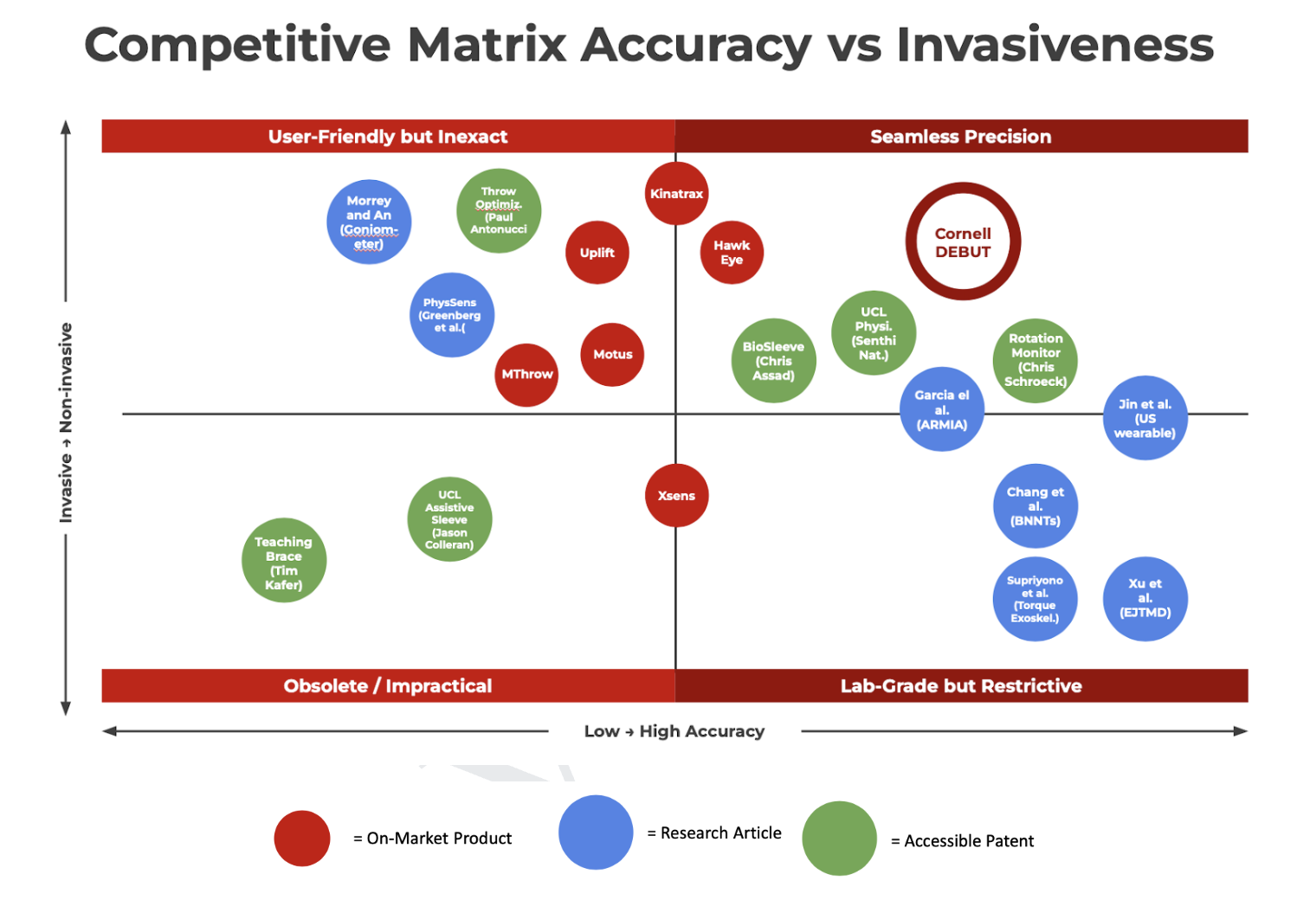
Lead project management and system engineering approach of a novel, Class II diagnostic device quantifying excess sports-related loading of the UCL (elbow ligament)
Outcomes/ Constributions
Defined product development standards for Cornell DEBUT project team with +200 technical onboarding slides and internal reports for design checkpoints
Organized renovation for shared prototyping and design laboratory space with +$2500 in new industry-level prototyping equipment
Manage 3 distinct technical teams with weekly deliverables in Click-Up
Wrote and compiled +100 pages of R&D, regulatory, and commercial documents for FDA Design History File consistent with for Class II standards
Lead athletic immersion program for defining +30 in-field mechanical, electrical, and software design constraints for initial prototypes
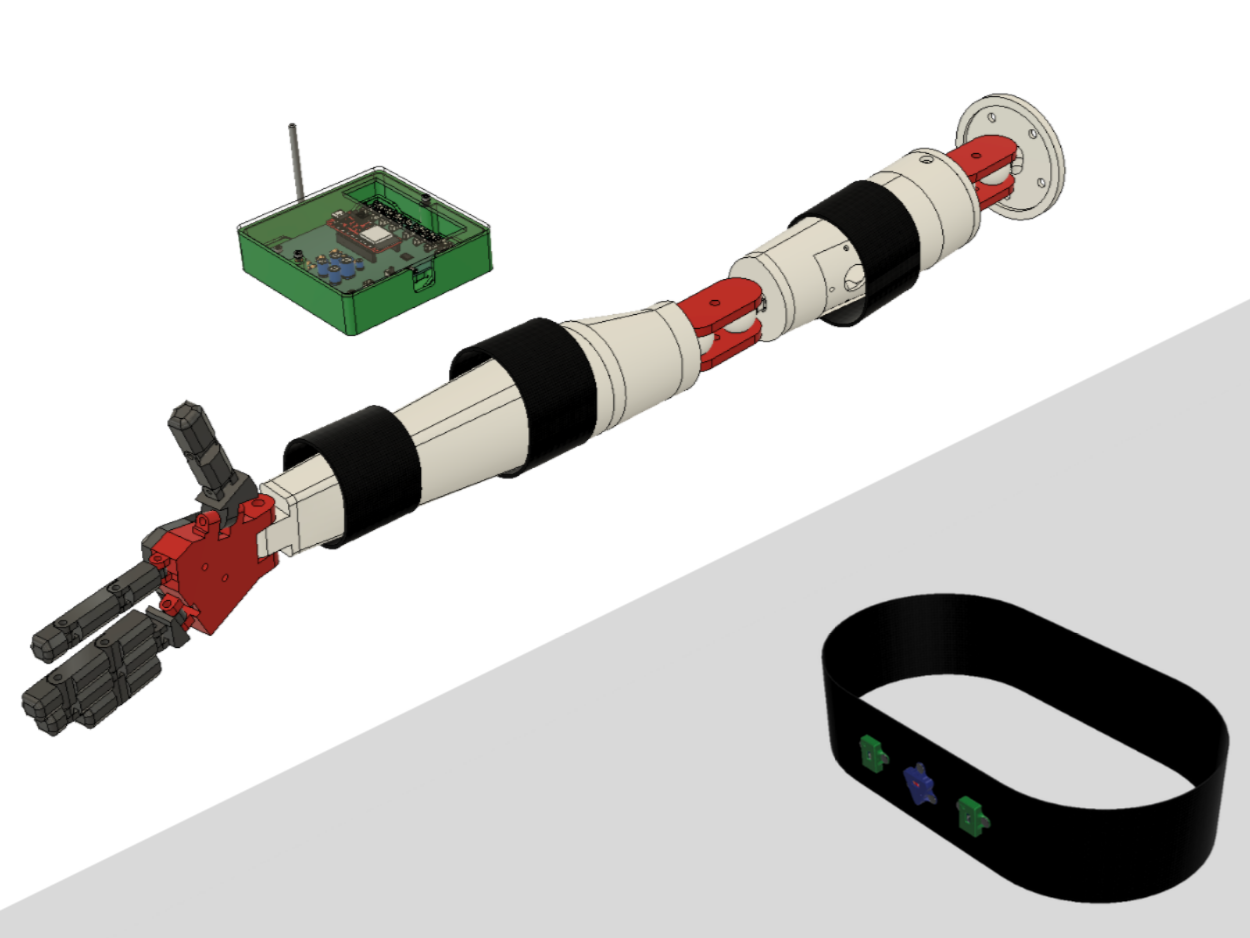
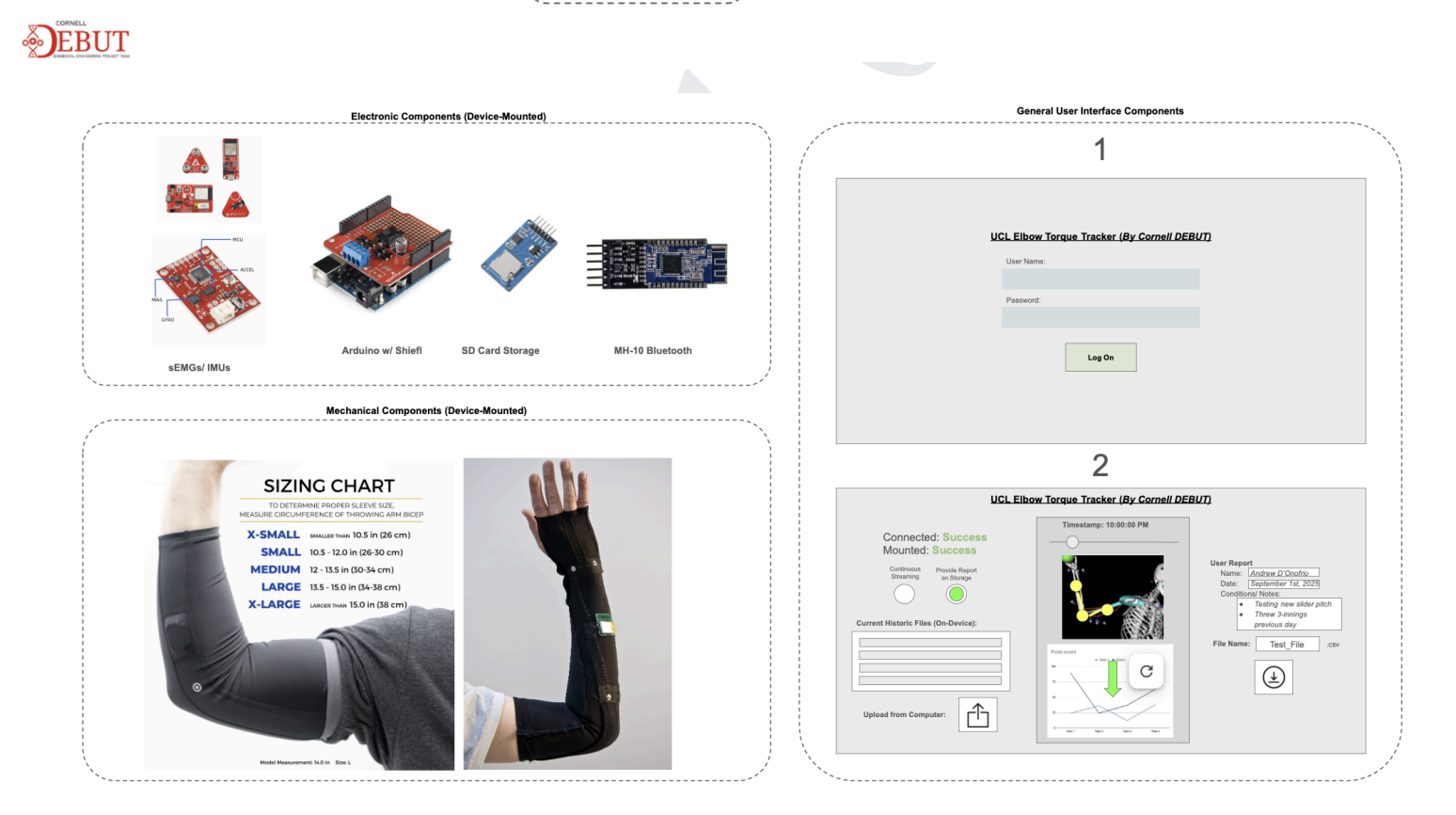
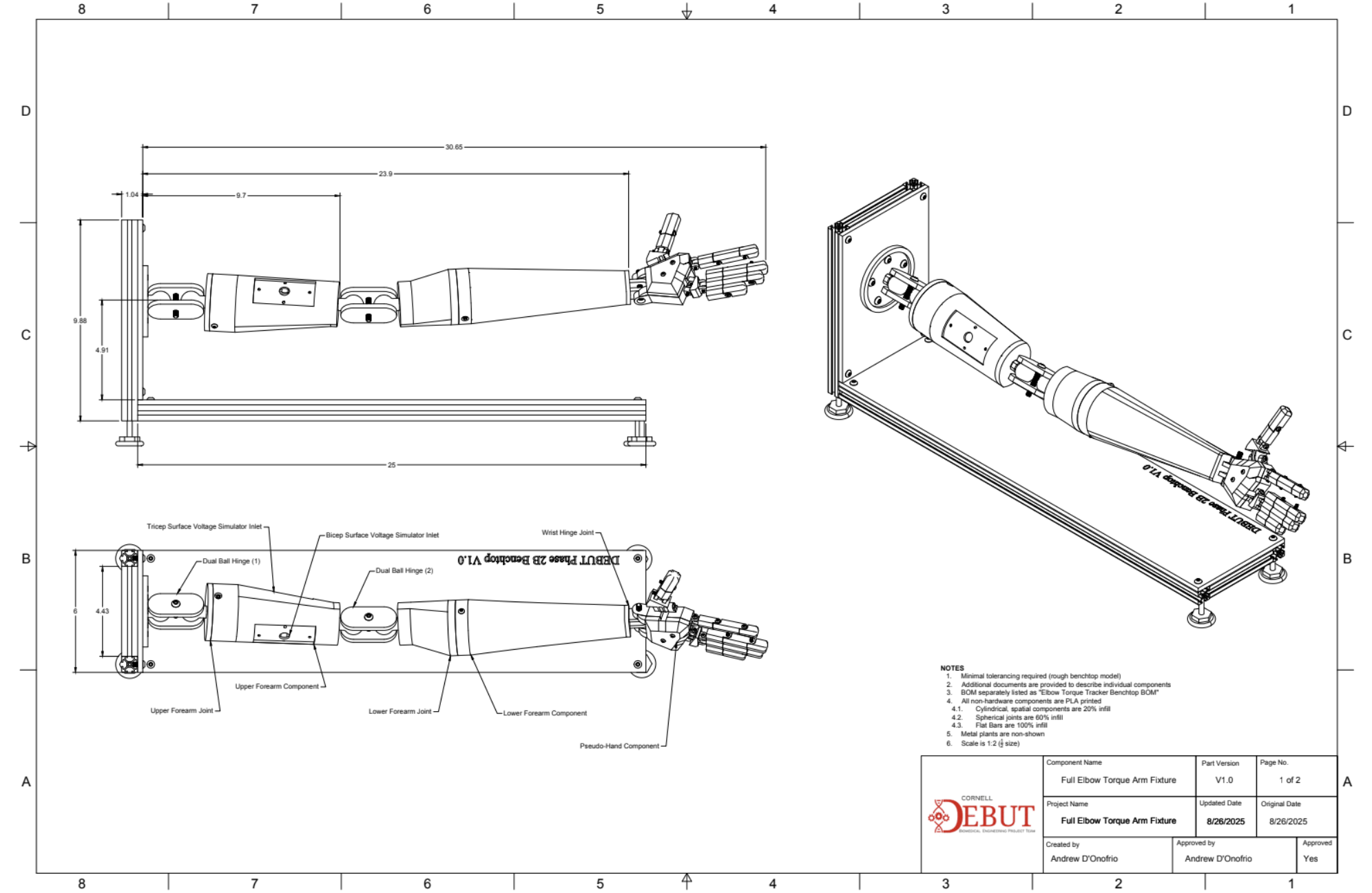
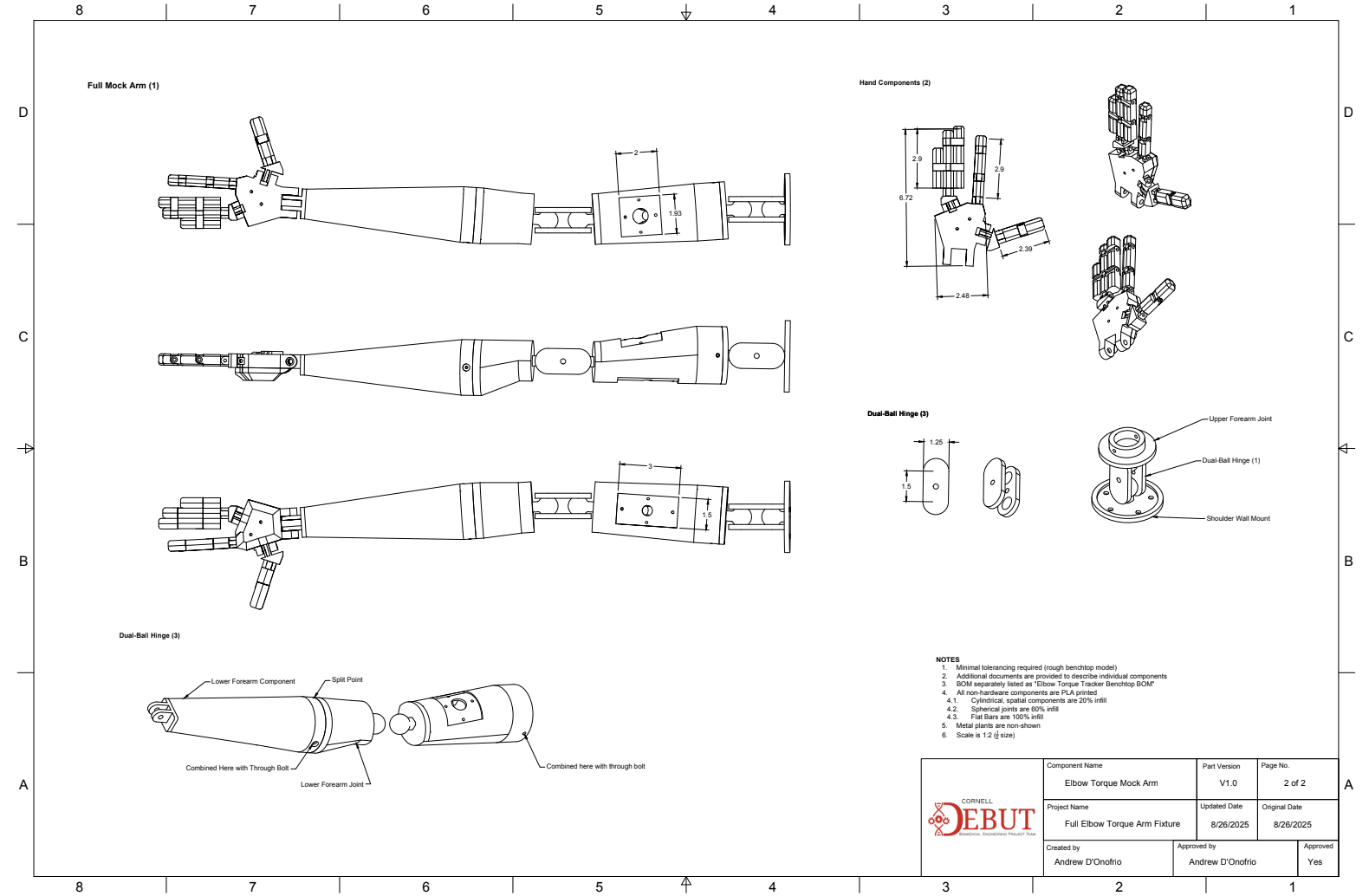
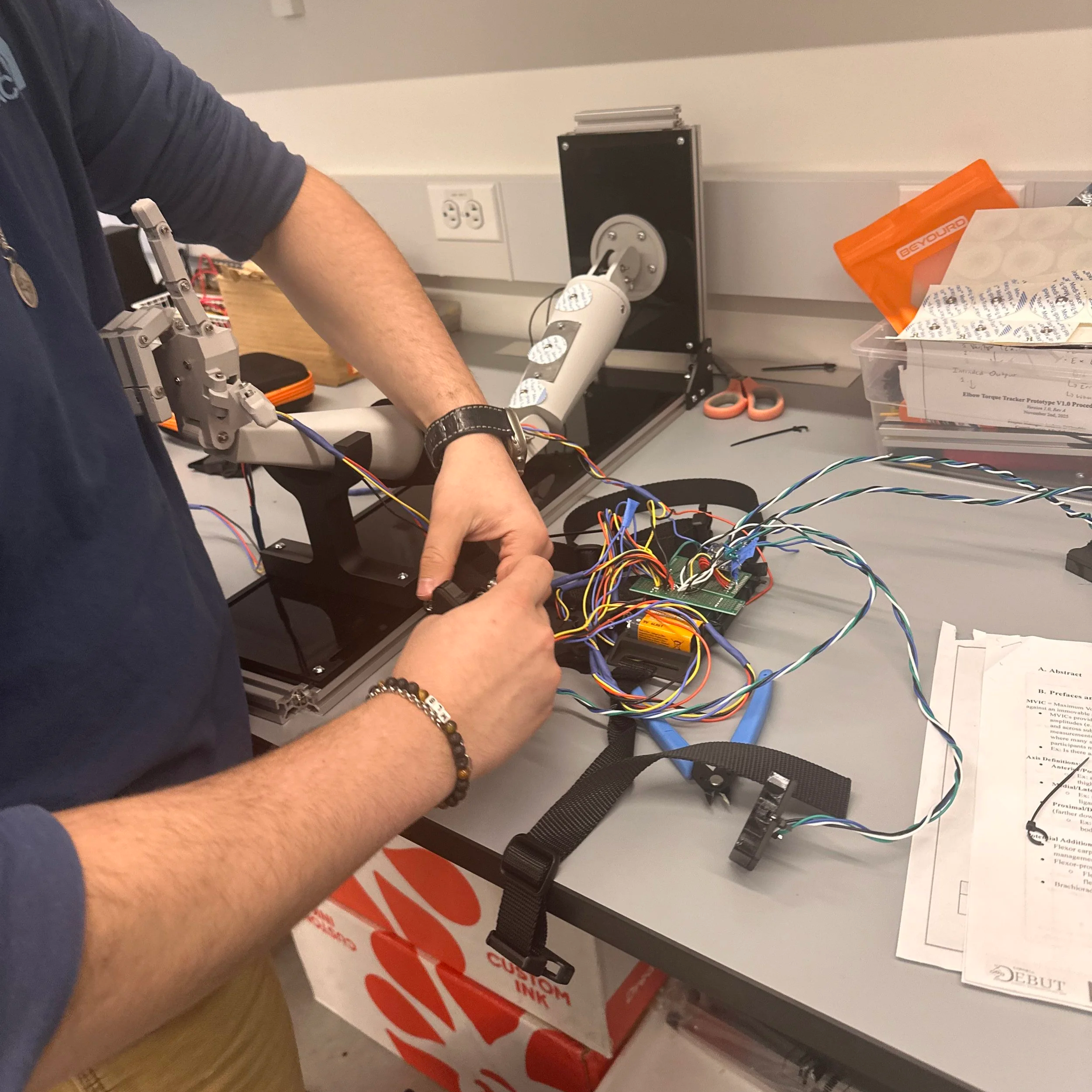
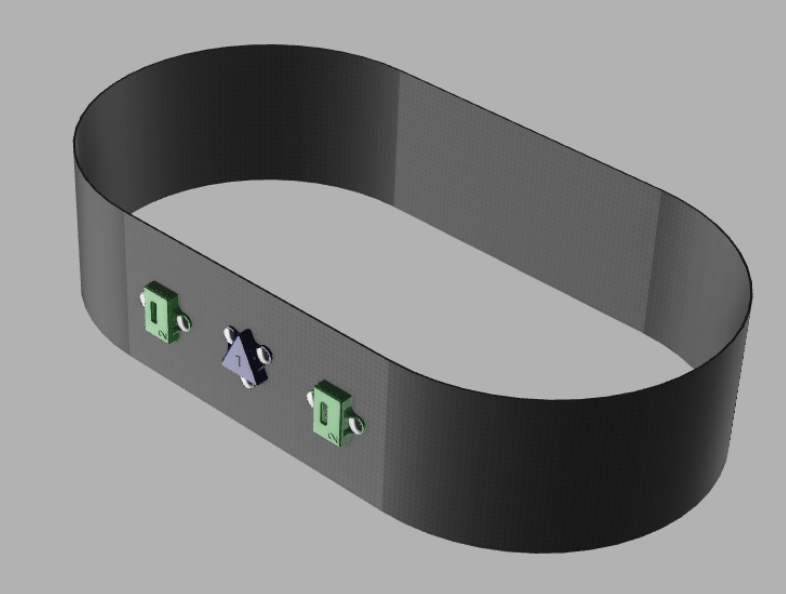
Designed and assembled full-scale wearable equipment in Fusion360 for operational sEMG and IMU equipment utilized in the V1.0 prototype
Translate pre-existing literature on Hills-Musculoskeletal model into a proprietary Python-based processing UI hosted in GitLab (ISO-compliant)
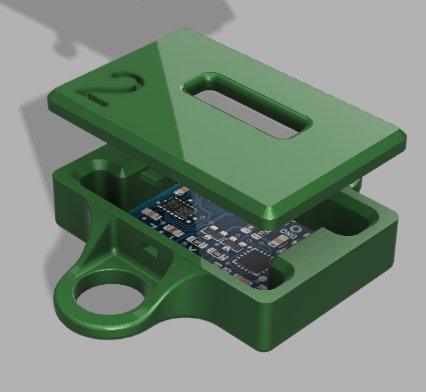
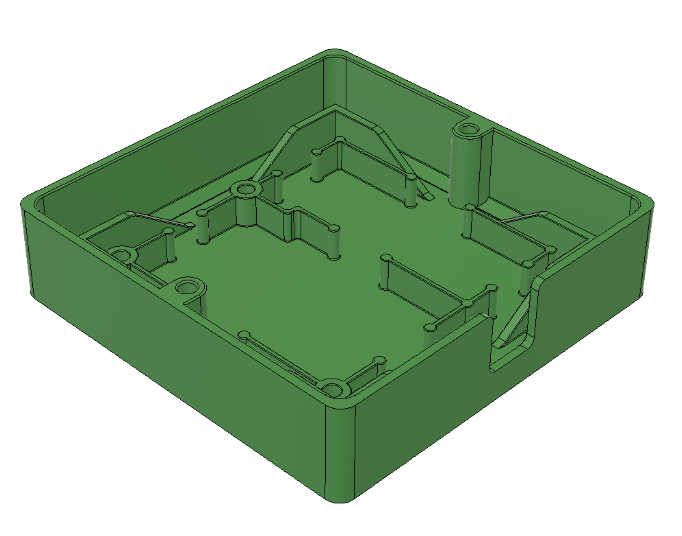
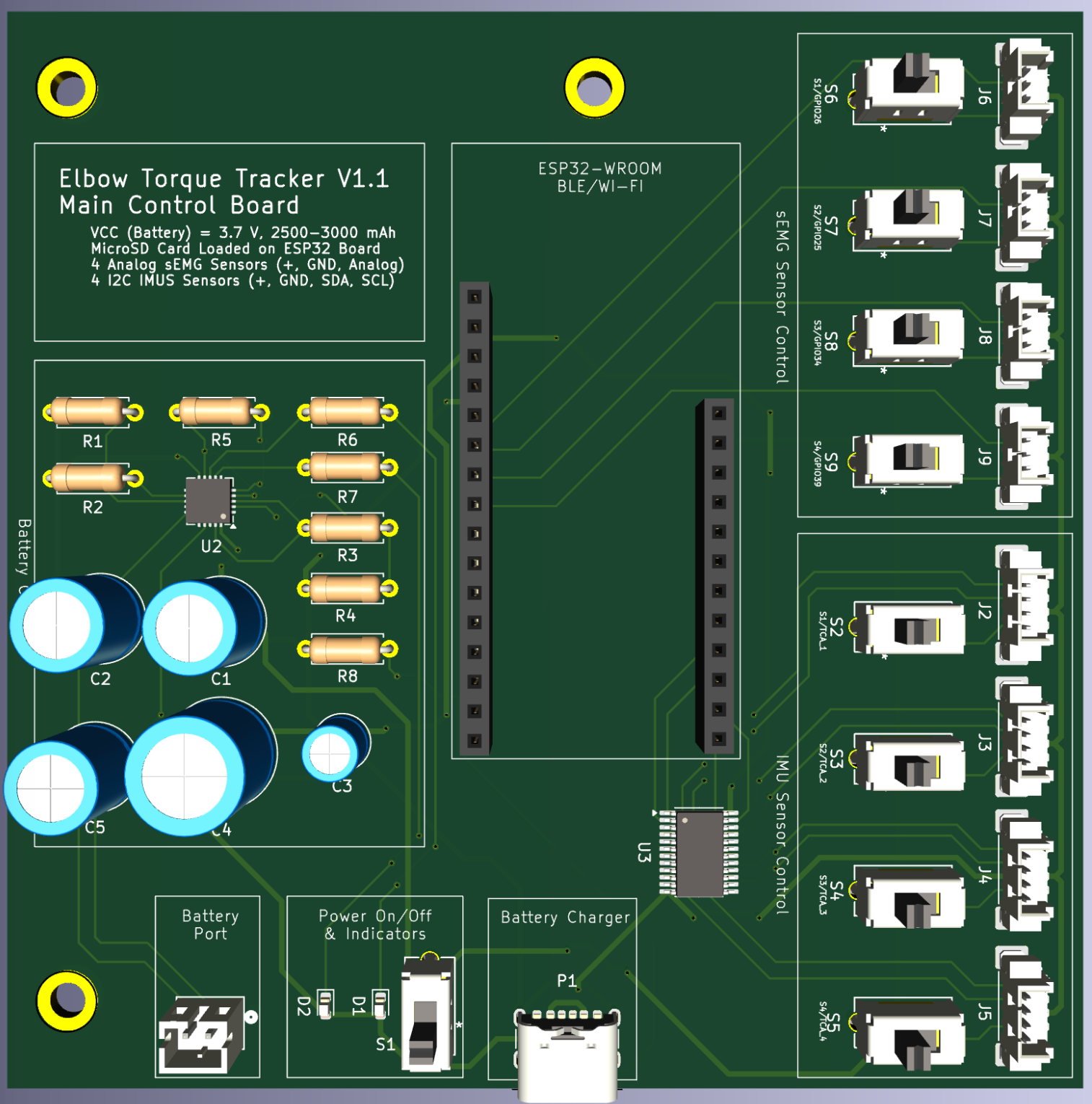
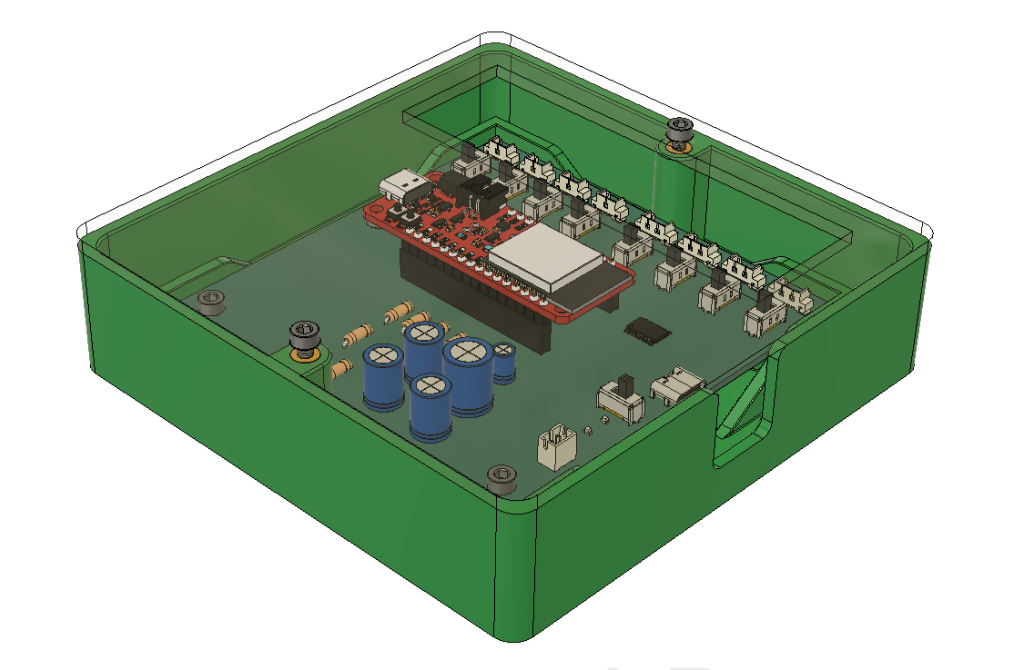
Designed 2-Layer main and peripheral PCB boards in KiCAD for V1.1 prototypes, with +10 chips connecting to 8 Molex and JST connectors
Relevant Skills
Systems engineering, project management, engineering leadership, technical training and onboarding, regulatory compliance and ISO-training, verification & validation testing, interpersonal conflict management, large-scale electromechanical design, Click-Up, GitLab/ GitHub, Google Sheets/ Excel
Background
The Elbow Torque Tracker device targets muscle groups and creates time-dependent models for arm-throwing motions to provide a real-time estimate of elastic stress of the UCL. The device consists of passive, electromechanical sensors that non-invasively fixes on the elbow region of a user’s dominant arm, and continuously streams muscle firing and positional information from a local CPU to a secondary UI downloaded through a mobile application. The UCL provides both technical and non-technical indicators for high elbow torque (and other stresses associated), such as color indicators for at-risk motions and in-depth description of individual data points.
Following the end of life for the SteadyStride project, I received the opportunity to lead a new biomedical project with a team of 12 engineers as a part of Cornell DEBUT. With the learnings from meeting with members of the NIH and Medtronic from the previous 2024 BMES Conference, our team was inspired to maintain industry quality in our R&D process at Cornell and push the potential for a start-up quality venture. After a few months of brainstorming, our team had converged on a novel diagnostic for managing UCL tears in MLB players with pre-existing research on muscle-firing rates. The following is a portion of the abstract on the initial product definition report:
Example Training Resources
Project Management (Documentation, Training, etc.)
As a project manager, I aimed to elevate two major aspects about the typical bio-entrepreneurial process for our team and distinguish among other college start-ups: extensive, iterative testing process and industry-level documentation. From experience, typical university-level products lack the proper testing and traceability to prove early-stage efficacy, which stem from unclear long-term timelines. This is mitigated for my team by creating a clear short, medium, and long term guidelines, providing new infrastructure/ internal requirements for documentation, and creating clear benchmarks for verification and validation success. As our new product development process began in 2025, I lead 5 distinct technical teams (electrical, mechanical, software, testing, client/regulatory) towards creating our first full-scale, novel prototype December 2025. This included the following management steps:
4 all-hands meetings defining project scope, market segment (IP search, competitive matrix, etc.), actionable items across technical groups (including 6-month plans), and a problem definition report
1 pre-DV and 1 alpha prototype design meetings to quantifiable rank major design requirements
+30 meetings technical meetings from August to December 2025 leading technical workshops, laboratory trainings, conducting device/ software root cause analysis, and integration meetings towards prototype V1.0 checkpoint
5 meetings with Cornell laboratory teaching staff to obtain independent access for motion capture studio
20 technical reports and protocols (primary author for ~25%) documenting full design process, acting of the culmination of +100 individual product action items
Future steps involve strategic planning for IRB-testing for validation, coordinating initial usability testing and feedback from key customer segment, and integrating additional feedback from customers into prototype V2.0.
Design History File Management
Testing Processes and Equipment
Testing procedures were planned prior to product development as a framework for major design checkpoints, specifically 1 sensor testing suites, 1 full-scale electromechanical testing suite, and 1 full-scale product testing suite (electromechanical & GUI). These tests were developed in context of CFR 890.1375 (Diagnostic electromyograph) and associated ISO standards, and set major requirements for device performance metrics, reliability, and usability in V&V testing. The following is an example of piece of verification equipment I developed for isolated sensor testing, and one testing SOP I wrote for systems testing for validation against typical valgus elbow torque methods:
Mock Arm Apparatus for Verification Testing
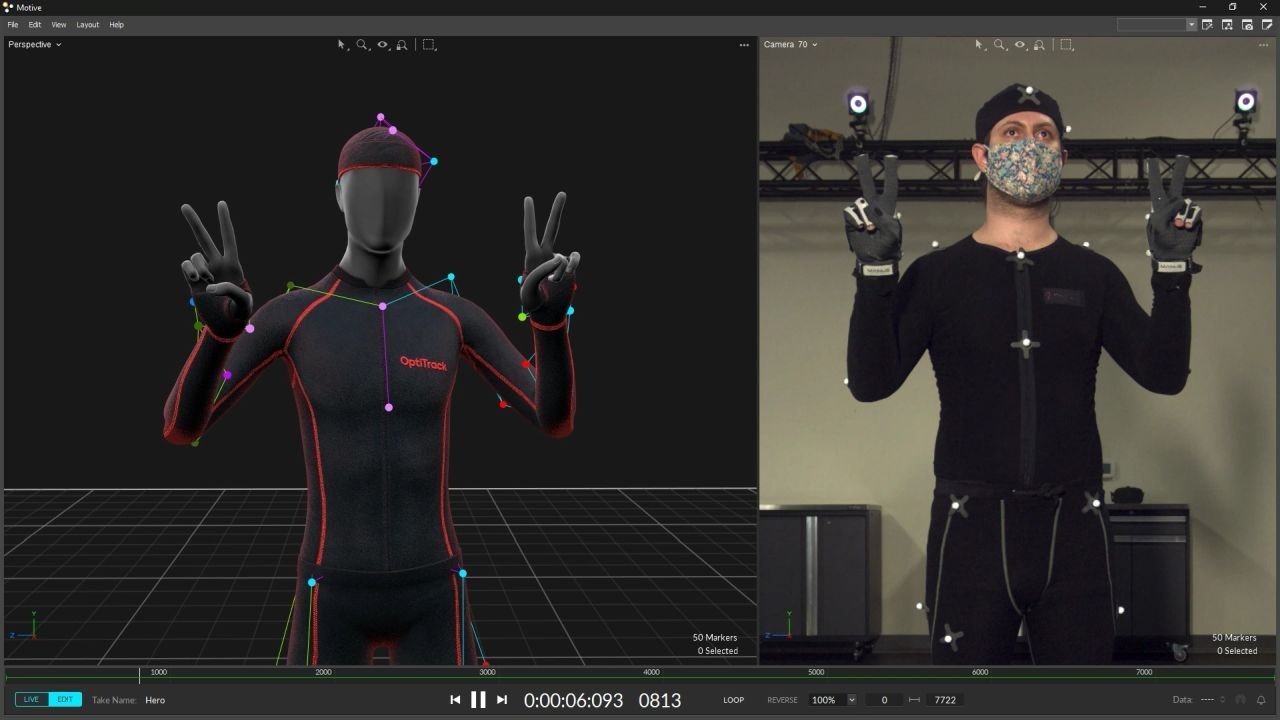
OptiTrack Motion Capture for Validation Testing
OptiTrack - Common Motion Capture style infrared tracking system, typically used for inverse kinematics for elbow torque tracking
Error estimation is conducted between the inverse kinematics approach with the OptiTrack system and the proprietary Elbow Torque Tracker (ETT) in order to determine experimental accuracy and tune full-scale details such as placement of sensors, usability, etc.
Athletic Immersion Program
Additionally, prior to initial designs, mechanical design requirements were defined through an internal athletic immersion program. Protocols were written to isolate particular TRIZ principles (“Theory of Inventive Problem Solving”) such as estimated length of moving object, weight of stationary object, force/ pressure, etc. These protocols were then tested against various arm-throwing sports including lacrosse, tennis and baseball to create 18 major design constraints required for a device with the intended scope. These are photos from the following experience:
Electromechanical Design Checkpoints
Currently, our product has reached the first major checkpoint for our development cycle (Prototype V1.0) and is currently underway for the second development checkpoint (Prototype V1.1). More specifically, Prototype V1.0 is an integration of major electrical component such as sensor interfacing with intended MCU, specifying power requirements for all components, relevant break-out boards soldered onto a protoboard, and automating software for MCU data collection. These components are fixed with basic mechanical casings that hold the form factors that future iterations will include. Prototype V1.1 will include an all-in-one “main” PCB for coordinating MCU and sensor interactions, “peripheral” for combining individual sensors, and improving casing designs.